Physiological Effect of Kinetin on Germination and
Successive Growth of Mesta [Hibiscus Sp.]
KHURSHID EQBAL AND S. K. PAL
University College of Agriculture, Calcutta University,
35 Ballygunge Circular Road, Calcutta 700019, India.
Abstract
The influence of kinetin on seed germination and seedling growth in three varieties of mesta, Hibiscus, namely, HC 583, HS 7910 and HS 4288, was investigated. Kinetin stimulated the germination of mesta seeds of all varieties only when treated with 1, 2 and 4 ppm. Treatments showed accelerating effect upto 4 ppm. Kinetin enhanced dry matter accumulation at lower concentrations but reduced it at higher concentrations. Seeds treated with kinetin, produced more expanded cotyledons than control seeds irrespective of mesta varieties considered. |
The cultivation of jute is restricted in eastern zone of India comprising the states of West Bengal, Tripura, Bihar, Assam, Orissa and some parts of Uttar Pradesh As a result, the production of jute fiber fell short of mill's requirement in the country. This situation ne-cessitated the cultivation of mesta and roselle (both are called mesta in India) which could easily be grown at wider climatic and soil conditions with much less care and attention.
In India, mesta contributes to 23.74% of total fiber production. Naturally, an attempt is necessary to push through the yield potential of mesta. Farmers are facing problems on lower percentage of viability and germinability of seeds. Thus the poor establishment of seedlings occurs because of the immature death of seedlings in early growth period (20 days). The present study was undertaken to elucidate the influence of kinetin on above attributes since this bioregulant is known to improve germinability and growth of seedlings in some other crops.

Hibiscuis cannabinus
Cytokinins play a key role in the life of higher plants. Earlier author (1) suggested that there might have been a chemical regular tor in plants which can stimulate the cell division. Other author (2) found that diffusates from phloem tissue could induce cell division in parenchyma. Using modern tissue-culture techniques, later workers (3) essentially repeat ted the experiment initially carried out by Haberlandt (2). They showed that a piece of vascular tissue cultured on the top of tobacco pith tissue could cause division of the pith cells, which could not be otherwise divided. These observations led to search for the pure substance which could induce cell division in a manner similar to that of the unknown substance or substances in vascular tissue.
 The first attempt to isolate the active material was made with aqueous extracts from tobacco stems which gave positive but inconsistent results (3). Because of this poor reproductivity, the investigators turned to other sources and found that coconut milk and malt extract (3), yeast extract and autoclaved DNA (4) were highly active. From autoclaved herring sperm DNA, the first crystal of a cell division inducing a substance was 6-furfuryl-aminopurine and it was named Kinetin (4). The name of the substance obviously was based on its property of causing cell division or cytokinesis in tobacco pith tissue. In 1956, the Wisconsin group proposed the generic term Kinin for the group of synthetic or naturally occurring substances which resembles kinetin in inducing cell division in certain excised tissues in the presence of the exogenous auxin. Ultimately, to avoid confusion on the nomenclature of Kinetin some workers (5) proposed that term Cytokinin be universally used as a generic name for the substances which promotes cell division and exerts other growth regulatory functions in the same manner as kinetin does and the terms Kinin or Phytokinin are now avoided for the sake of confusion. The term kinetin of course, is reserved for one specific cytokinin, namely 6-furfuryl-aminopurine.
Cytokinins have a broad spectrum regulatory effects, inducing effects on dormancy, germination, growth rate, rooting, flower initiation, sex expression, fruit set, fruit growth, tuberisation, senescence and abscission.
Methods
Seeds of mesta (Hibiscus sp.) were collected from the Head, Division of Plant Breeding and Genetics, Jute Agricultural Research Institute (ICAR), Nilgunj, Barrackpore. Three varieties of mesta such as HS 7910, HS4288 and HC583 were selected for this investigation. The first two varieties belong to the species H. subdarijfa and the last one belongs to the species H, cannabinus. The experiments were conducted in the plant physiology laboratory, University College of Agriculture, Calcutta University.
Seed Viability Testing:
The seeds were surface sterilized with 1% mercuric chloride solution for 30 minutes and were thoroughly washed for 10 times with glass-distilled water. The viability of the seeds was tested by 2, 3, 5-triphenyl tetrazolium chloride (TTC). Five hundred milligram of TTC was dissolved in 4 drops of absolute alcohol and the volume was made upto 100 ml with glass-distilled water. This solution was stored in refrigerator in black box ; 0.5% solution of TTC was used for the determination of viability of seeds. Fifty seeds were soaked in distilled water for 12 hours and placed in each petridish. Each seed was cut longitudinally into two halves, in such a a manner so that each half should have contained a part of embryo. Two halves of each seed were kept in two different petridishes. Then these were treated with TTC and kept in dark chamber for half an hour. TTC was reduced during respiration forming triphenyl formazon, a red insoluble dye. Therefore, the viable seeds (respiring seeds) only got the red color. The number of colored seeds was counted. This test was repeated every 15-day intervals.
Effect of Kinetin on Germination:
Kinetin was used at 1,2, 4, 6, 8 and 10 ppm. The surface sterilized seeds were treated with these concentrations of kinetin for 12 hours. Seeds were germinated in petridishes, lined with filter paper and imbibed with distilled water. Each petridish contained 50 seeds. Seeds were germinated only in glass distilled water considered as control for necessary comparison. There were three replicates for each treatment.
Effect of Kinetin on Seedling Growth:
Normal sand culture method was adopted for the the growth of seedlings. Sand was sterilized with 1% HC1 for overnight and was washed thoroughly with water for about 30 times (until the pH of the sand becomes neutral). The sterilized sand was then filled in small polythene pots. Surface sterilized seeds, treated with kinetin, were sown in the pots at the rate of 15 seeds each. Seeds soaked in distilled water were considered as control. The pots were watered properly with glass-distilled water. On every third day, they were supplied Hoagland's nutrient solution (Version II). Sampling was done after 5 and 10 days of sowing. The length of hypocotyl and root, fresh weight, dry weight and cotyledon area were measured. For dry weight experiment, plants were kept in an oven for 72 hours at 65±1°C. The cotyledon area was measured with the help of constant by using the following formula :
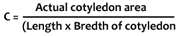
The actual leaf area was measured by a planimeter. After sampling of a large number of cotyledons, a mean value of 0.74 was found as the constant for mesta cotyledons.
Results
The experimental observations are presented in Tables 1-6 and Figures 1-5. The presowing treatment with kinetin stimulated the process of germination upto a concentration of 8 ppm and inhibited at 10 ppm (Fig. 2).
Table - 1:
| Effect of kinetin on the seedling growth of Hibiscus cannabinus Var HC 583 |
| Treatment |
5 days after sowing |
10 days after sowing |
Hypocotyle length
(cm) |
Root length
(cm) |
Hypocotyle length
(cm) |
Root length
(cm) |
| Control |
1
2
3 |
9.08
±0.39
(100) |
5.9
±0.63
(100) |
13.74
±0.78
(100) |
7.15
±0.54
(100) |
| 1 ppm |
1
2
3 |
11.53
±0.42
(126.98) |
6.3
±0.35
(106.78) |
114.85
±0.47
(108.08) |
7.75
±0.46
(108.39) |
| 2 ppm |
1
2
3 |
12.27
±0.39
(135.13) |
7.04
±0.28
(119.32) |
15.32
±0.51
(111.50) |
8.50
±0.49
(118.88) |
| 4 ppm |
1
2
3 |
12.01
±0.37
(132.26) |
7.06
±0.32
(119.66) |
16.09
±0.44
(117.10) |
9.10
±0.68
(127.27) |
| 6 ppm |
1
2
3 |
11.32
±0.49
(124.67) |
6.16
±0.17
(104.41) |
14.0
±0.58
(101.89) |
7.36
±0.58
(102.94) |
| 8 ppm |
1
2
3 |
9.35
±0.48
(102.97) |
5.84
±0.55
(98.98) |
13.44
±0.6
(97.81) |
7.01
±0.34
(98.04) |
| 10 ppm |
1
2
3 |
9.05
±0.40
(99.67) |
4.93
±0.40
(83.56) |
13.13
±0.61
(95.56) |
6.22
±0.34
(86.99) |
| 1 = Mean, 2 = ± S.E. , 3 = % over control
|
Table - 2:
| Effect of kinetin on the seedling growth of Hibiscus cannabinus Var HC 583 |
| Treatment |
5 days after sowing |
10 days after sowing |
Fresh Weight
(mg) |
Dry Weight
(mg) |
Fresh Weight
(mg) |
Dry Weight
(mg) |
| Control |
1
2
3 |
267.74
±14.33
(100) |
13.88
±0.80
(100) |
314.41
±20.46
(100) |
16.97
±0.77
(100) |
| 1 ppm |
1
2
3 |
356.25
±14.59
(133.06) |
15.52
±0.85
(111.82) |
379.44
±20.11
(120.68) |
18.82
±1.12
(110.90) |
| 2 ppm |
1
2
3 |
367.47
±9.66
(137.25) |
18.59
±0.65
(133.93) |
392.58
±14.16
(124.86) |
20.89
±1.49
(123.10) |
| 4 ppm |
1
2
3 |
382.71
±7.90
(142.94) |
19.08
±0.49
(137.46) |
414.37
±28.27
(131.79) |
21.79
±1.49
(128.40) |
| 6 ppm |
1
2
3 |
301.47
±16.66
(112.60) |
18.02
±0.86
(129.83) |
340.57
±7.87
(108.32) |
20.32
±1.46
(119.47) |
| 8 ppm |
1
2
3 |
266.32
±15.11
(99.00) |
17.73
±0.66
(127.74) |
335.03
±23.63
(106.56) |
20.47
±1.85
(120.62) |
| 10 ppm |
1
2
3 |
256.21
±9.43
(95.69) |
12.82
±1.05
(92.36) |
284.65
±21.49
(90.53) |
16.43
±0.62
(96.82) |
| 1 = Mean, 2 = ± S.E. , 3 = % over control
|
Table - 3:
| Effect of kinetin on the seedling growth of Hibiscus subdariffa Var HS 7910 |
| Treatment |
5 days after sowing |
10 days after sowing |
Hypocotyle length
(cm) |
Root length
(cm) |
Hypocotyle length
(cm) |
Root length
(cm) |
| Control |
1
2
3 |
8.33
±0.43
(100) |
4.55
±0.44
(100) |
11.42
±0.52
(100) |
6.31
±0.37
(100) |
| 1 ppm |
1
2
3 |
9.51
±0.34
(114.17) |
5.5
±0.21
(120.88) |
12.43
±0.32
(108.84) |
6.77
±0.30
(107.29) |
| 2 ppm |
1
2
3 |
10.03
±0.52
(120.41) |
6.26
±0.22
(137.58) |
13.04
±0.26
(114.19) |
7.56
±0.26
(119.01) |
| 4 ppm |
1
2
3 |
10.47
±0.32
(125.59) |
6.54
±0.33
(143.74) |
14.97
±0.49
(131.09) |
7.88
±0.24
(124.88) |
| 6 ppm |
1
2
3 |
10.98
±0.35
(131.81) |
6.05
±0.11
(132.97) |
12.30
±0.46
(107.71) |
7.02
±0.16
(111.25) |
| 8 ppm |
1
2
3 |
8.04
±0.49
(96.52) |
4.84
±0.13
(106.37) |
10.73
±0.29
(93.96) |
6.11
±0.14
(96.83) |
| 10 ppm |
1
2
3 |
7.65
±0.35
(91.84) |
3.95
±0.19
(86.81) |
10.12
±0.69
(88.62) |
5.6
±0.39
(88.75) |
| 1 = Mean, 2 = ± S.E. , 3 = % over control
|
Table - 4:
| Effect of kinetin on the seedling growth of Hibiscus subdariffa Var HS 7910 |
| Treatment |
5 days after sowing |
10 days after sowing |
Fresh Weight
(mg) |
Dry Weight
(mg) |
Fresh Weight
(mg) |
Dry Weight
(mg) |
| Control |
1
2
3 |
125.97
±4.73
(100) |
7.26
±0.42
(100) |
173.45
±12.92
(100) |
9.79
±0.46
(100) |
| 1 ppm |
1
2
3 |
137.7
±4.69
(109.31) |
8.36
±0.28
(115.15) |
188.62
±5.53
(108.75) |
10.74
±1.12
(109.70) |
| 2 ppm |
1
2
3 |
149.5
±5.40
(118.68) |
9.83
±0.40
(135.40) |
193.42
±5.87
(115.51) |
11.03
±0.45
(112.67) |
| 4 ppm |
1
2
3 |
152.27
±6.96
(120.88) |
10.79
±0.52
(148.62) |
207.1
±12.48
(119.40) |
11.43
±0.39
(116.75) |
| 6 ppm |
1
2
3 |
147.04
±6.23
(116.73) |
9.25
±0.55
(127.41) |
182.7
±6.00
(105.33) |
10.04
±0.34
(102.55) |
| 8 ppm |
1
2
3 |
130.24
±8.64
(103.39) |
8.44
±0.36
(116.25) |
163.98
±4.68
(94.54) |
9.64
±0.38
(98.47) |
| 10 ppm |
1
2
3 |
125.27
±4.54
(99.44) |
7.83
±0.32
(107.86) |
158.79
±6.51
(91.55) |
9.19
±0.46
(93.87) |
| 1 = Mean, 2 = ± S.E. , 3 = % over control
|
Table - 5:
| Effect of kinetin on the seedling growth of Hibiscus subdariffa Var HS 4288 |
| Treatment |
5 days after sowing |
10 days after sowing |
Hypocotyle length
(cm) |
Root length
(cm) |
Hypocotyle length
(cm) |
Root length
(cm) |
| Control |
1
2
3 |
8.53
±0.42
(100) |
4.29
±0.39
(100) |
11.65
±0.43
(100) |
4.63
±0.25
(100) |
| 1 ppm |
1
2
3 |
9.05
±0.79
(108.38) |
5.57
±0.57
(129.84) |
12.53
±0.53
(106.01) |
6.54
±0.71
(141.25) |
| 2 ppm |
1
2
3 |
9.37
±1.11
(112.22) |
5.66
±0.78
(131.93) |
13.19
±0.61
(113.22) |
6.95
±1.83
(150.11) |
| 4 ppm |
1
2
3 |
10.42
±0.26
(124.79) |
6.28
±0.47
(146.39) |
13.85
±1.01
(118.88) |
7.07
±0.73
(152.70) |
| 6 ppm |
1
2
3 |
6.21
±0.27
(74.37) |
5.43
±0.62
(126.57) |
11.4
±0.22
(97.85) |
6.42
±0.56
(138.66) |
| 8 ppm |
1
2
3 |
6.62
±0.37
(79.25) |
4.89
±0.61
(113.95) |
10.22
±0.62
(87.73) |
5.34
±0.52
(115.33) |
| 10 ppm |
1
2
3 |
5.61
±0.48
(67.19) |
4.55
±0.75
(106.06) |
9.44
±0.55
(81.03) |
4.88
±0.60
(105.40) |
| 1 = Mean, 2 = ± S.E. , 3 = % over control
|
Table - 6:
| Effect of kinetin on the seedling growth of Hibiscus subdariffa Var HS 4288 |
| Treatment |
5 days after sowing |
10 days after sowing |
Fresh Weight
(mg) |
Dry Weight
(mg) |
Fresh Weight
(mg) |
Dry Weight
(mg) |
| Control |
1
2
3 |
150.28
±11.94
(100) |
8.35
±0.35
(100) |
161.71
±6.94
(100) |
9.45
±0.28
(100) |
| 1 ppm |
1
2
3 |
164.74
±11.09
(109.62) |
9.59
±0.26
(114.85) |
178.2
±8.51
(110.20) |
10.95
±0.51
(115.87) |
| 2 ppm |
1
2
3 |
186.15
±9.3
(123.87) |
10.00
±0.33
(119.76) |
199.6
±7.1
(123.43) |
11.75
±0.45
(124.34) |
| 4 ppm |
1
2
3 |
152.27
±6.96
(120.88) |
10.79
±0.52
(148.62) |
207.1
±12.48
(119.40) |
11.43
±0.39
(116.75) |
| 6 ppm |
1
2
3 |
146.67
±11.48
(97.60) |
8.40
±0.28
(100.60) |
169.51
±11.05
(104.82) |
9.67
±0.34
(102.33) |
| 8 ppm |
1
2
3 |
132.4
±12.30
(88.10) |
8.10
±0.36
(97.01) |
151.76
±7.23
(93.85) |
9.07
±0.41
(95.98) |
| 10 ppm |
1
2
3 |
115.66
±17.89
(76.96) |
8.01
±0.19
(95.93) |
144.89
±7.10
(89.60) |
8.53
±0.23
(90.26) |
| 1 = Mean, 2 = ± S.E. , 3 = % over control
|
Experiments on HC583
Kinetin showed significant enhancement in length of hypocotyl and root at 2,4 and 6 ppm and reduction at 10 ppm (Table 1). It was also observed that kinetin increased both fresh weight and dry weight of seedlings upto 8 ppm concentration and reduced both these attributes at 10 ppm (Table 2).
The average maximum fresh weight and dry weight of seedlings was found to be 382.71 mg and 19.08 mg per seedling respectively, after 5 days of sowing at 4 ppm (Table 2). The area, of cotyledon was observed to be increased by kinetin gradually with the increase in concentration upto 4 ppm, but reduced thereafter (Fig. 3). However, the values obtained at 6, 8 and 10 ppm were found significant over control. The influence of kinetin on elongation growth of hypocotyl and root (Table 1), fresh weight and dry matter accumulation (Table 2) and area of cotyledon was found to be persistent at 10 days after sowing.
Experiment on CV HS7910
The presowing treatment with kinetin increased the length of hypocotyl and root gradually with increase in concentration upto 6 ppm and decreased both these attributes at 8 and 10 ppm (Table 3) in H. subdariffa (CV HS7910). The fresh weight and dry matter accumulation of seedlings were enhanced by kinetin at 1,2, 4, 6 and 8 ppm and were reduced at 10 ppm (Table 4). Kinetin caused significant radial expansion of cotyledons upto 8 ppm (Fig. 4). The maximum radial expansion of cotyledons was found 105.76 sq mm and 126.33 sq mm in 5 days and 10 days old seedlings respectively. The promotion in elongation growth of hypo- cotyl and root was also observed in 10 days old seedlings in this variety (Table 3).
Experiment on CV HS4288
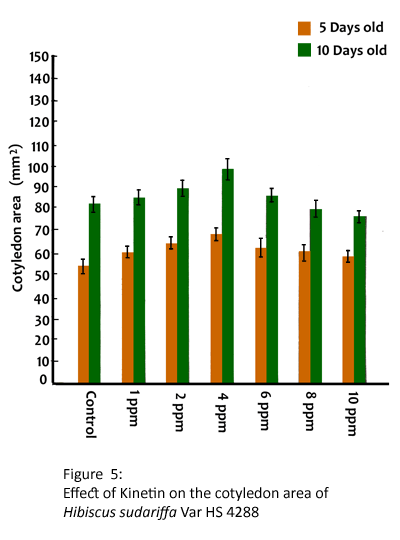
The exogenous application of kinetin stimulated the elongation growth of hypocotyl and root at 1, 2 and 4 ppm and drastically reduced at 6, 8 and 10 ppm in HS4288 (Table 5). The maximum elongation growth of hypocotyl in 5 and 10 days was found to be 10.42 and 13.85 cm respectively. It is also found that the pretreatment with kinetin enhanced the fresh weight and dry matter accumulation in seedlings upto 4 ppm and reduced at 10 ppm (Table 6). Further, the highest dry matter accumulation in CV HS4288 was found to be 10.80 and 12.84 mg per seedling at 5 and 10 days respectively. Kinetin also increased the area of cotyledons at 1, 2, 4 and 6 ppm and reduced at 8 and 10 ppm (Fig. 5) in 5-day old seedlings. A similar trend was observed in 10-day old seedlings.
Varietal Difference
The experimental findings exhibit the varietal differnce in seedling growth of mesta. H. camabinus, HC 583 showed best sensitivity to kinetin in terms of seedling elongation growth which was found to be 12.01 and 16.09 cm of hypocotyl in 5-day and 10-day old seedlings respectively. For CV HS4288, the same was found to be 10.42 and 13.85 cm in 5-day and 10-day old seedlings respectively. The maximum dry matter accumulation for HC583, HS7910 and HS4288 was found to be 21.79, 11.43 and 12.84 mg respectively per seedling at 4 ppm of kinetin. Evidently, the variety HC583 responded best to kinetin treatment followed by HS7910 and HS4288.
Discussion
The experimental results reveal the physiological response of three varieties of mesta to kinetin at different concentrations. Kinetin, at 4 ppm concentration, showed the highest percentage of germination irrespective of varieties considered. The trend of germination promotion was in accordance with those reported for Arachis hypogea (6, 7), Cajanus cajan (8) and Zea mays (9).
Tables 1, 3 and 5 show that the kinetin increased the elongation growth of hypocotyl and root upto 4 ppm. Thereafter, it did not influence growth. Such trend of elongation growth was also reported in Arachis hypogea (7), Bry- ophyllum tubiflorum (10) and Pisum sativum (11). Thus, the findings confirmed the action of kinetin in inducing the cell division (5). Even, the inhibition of elongation growth at higher concentration of kinetin was observed, which indicates that the kinetin is a growth regulator that has the property to induce inhibition of cell division or elongation at the higher concentration. Similar trend of inhibition of cell elongation and cell division affected by kinetin at higher concentration was also reported for Pisum sativum (11) and Lycopersecum escu- lentum (12).
Apparently, the exogenous application of kinetin was responsible for the cell elongation and cell division (13, 14) of the seedlings of mesta, which has led to the increase of fresh weight and dry matter accumulation. Thus, the findings obtained from this investigation coroborate the observations in various other plants such as Cajanus cajan (8), Zea mays (9) and Pisum sativum (11). Moreover, the kinetin significantly increased the area of cotyledons in all three varieties of mesta considered in this investigation. Kinetin was responsible, in elongation growth of cell which was maximum at 4 ppm.
References
- Wiesner J. 1982. Ein Etemen tars truk- tur Und das wachstum der lebender subs- taz. Holder, Vienna.
- Haberdandt G. 1913. Zur Physiologic der zelteilung. Sitzungsber. K. Preuss. Akad. Wiss. 1913 : 318-345.
- Jablonski J. R. and F. Skoog. 1954. Cell enlargement and cell division in excised tobacco pith tissue. Physiol. Plant. 7 : 16-24.
- Miller C. O., F. Skoog, F. S. Okumura. M. H. Von Saltza and F. M. Strong.1955. Structure and synthesis of kinetin,
J. Am. Chem. Soc. 77 : 2662-2663.
- Skoog F. and C. O. Miller. 1957. Chemical regulator of growth and organ formation in plant tissues cultured in vivo. Symp. Soc. Exo. Biol. 11 : 118-131.
- Ketring D. L. and P. W. Morgan. 1971. Physiology of oil seeds II. Dormancy release in Virginia type peanut seeds by plant growth regulators. Plant. Physiol. 47 : 488-492.
- Joshi R. K" S. D. Mishra and B. K. Gaur. 1978. Release of dormancy in Trombay groundnut seeds by plant growth regulators, Acta Botanica Indica 6 : 7-15.
- Mehta P. M., M. T. Jacob and B. V. N. Rao. 1974. The effects of ascorbic acid, kinetin, coumarin and their combination on the germination, growth and enzyme catalase of Cajanus cajan var. selection 4-1A. Geobios 1 : 53-59.
- Bhatnagar V. K. and G. R. Rastogi. 1980. Effect of some growth regulators on Zeamays. Geobios 7 : 183-184.
- Bisaria A. K. 1981. Influence of chloro- flurenol and its combination with IBA, GA and kinetin on regenration of epi- phyllous buds in Bryophyllum tubiflorum. Acta Botanica Indica 9 : 252-257.
- Nandwal A. S. and S. Bharati. 1982. Effect of kinetin and IAA on growth, yield and nitrogen fixing efficiency of nodule in pea (Pisum sativum). Indian J. Plant Physiol. 25 : 358-363.
- Goel A. K. and M. S. Tayl. 1984. Phytotoxicity of carbofuran and aldicarb and its antagonism with growth hormones on germination, seedling growth and amylase activity. J. Indian Bot. Soc. 63 : 425-430.
- Torrey J. G. 1961. Kinetin as trigger for mitosis in mature endomitotic plant cell. Exp. Cell Res. 23 : 281-299.
- Leonard N" S. M. Hecht, F. S. Skoog and R. Y. Schnitz. 1968. Cytokinins : synthesis of 6 (3-methyl-3 butenylamino)- 9-8-D-ribofuranosylphurine and the effect of side-chain unsaturation on the biological activity of isopentyl aminopurine and their ribosides. Proc. Natl. Acad. Sci. (US) 59 : 15-21.
| This research work was done in 1984 by Khurshid Eqbal under the guidance of Prof. S. K Pal, University College of Agriculture, Calcutta University. Experiments were conducted at Division of Plant Breeding and Genetics, Jute Agricultural Research Institute (ICAR), Nilgunj, Barrackpore and Deptt. of Plant Physiology, University College of Agriculture, Calcutta University, 35 Ballygunge Circular Road, Calcutta 700019, India. |

| |